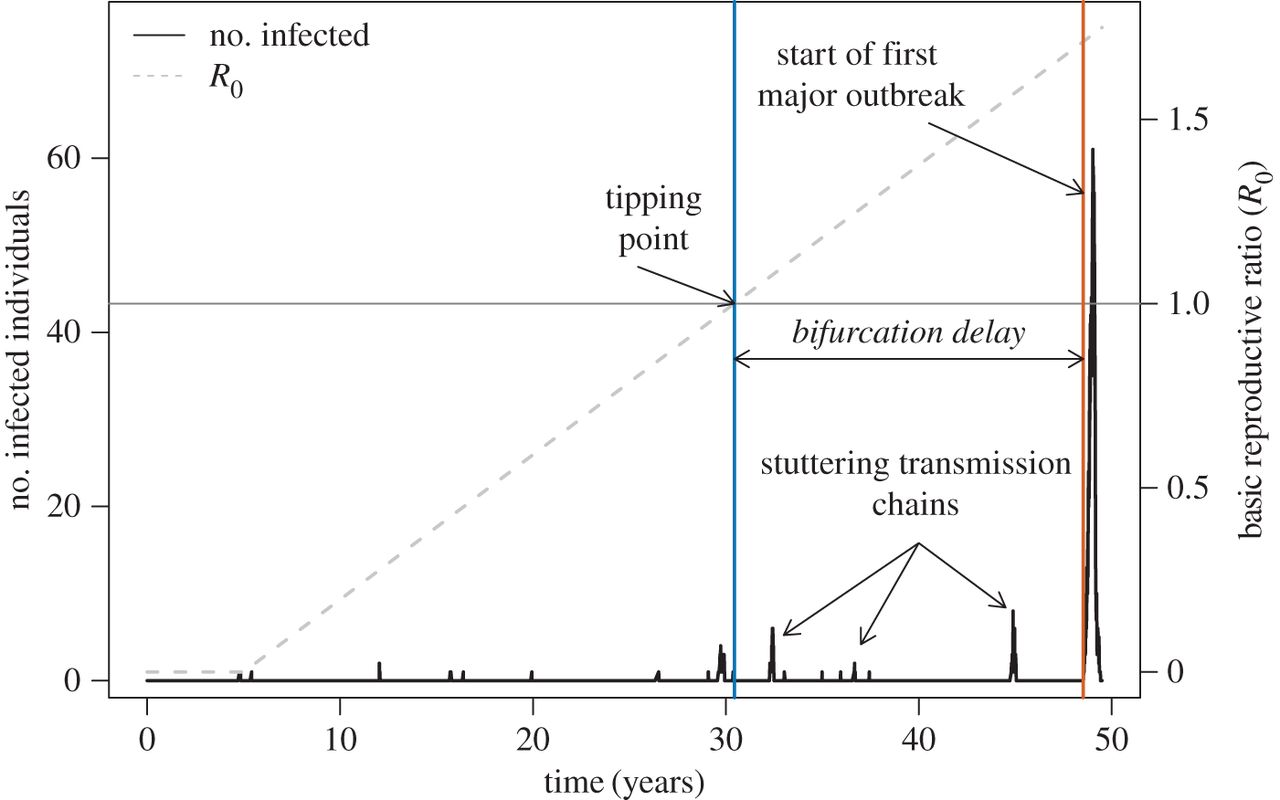
Led by Chris Dibble, we recently published a paper in JRS Interface that asked the question, “As R0 increases through 1, how long until a disease outbreak?”. Many systems have slowly increasing parasite fitness whether it’s through parasite evolution, demographic susceptible recruitment, or abandonment of vaccination (sweep rate). Susceptible populations are also regularly challenged with infectious individuals that have the potential to ‘spark’ an outbreak (spark rate). We integrated these two rates with epidemiology models and survivorship theory (which characterizes time to an event) to establish the waiting time to infectious disease emergence. We demonstrated that this time is influenced by factors such as infectious period, meaning that different infectious disease systems can cause outbreaks sooner after R0 exceeds 1, than others.
Category Archives: Uncategorized
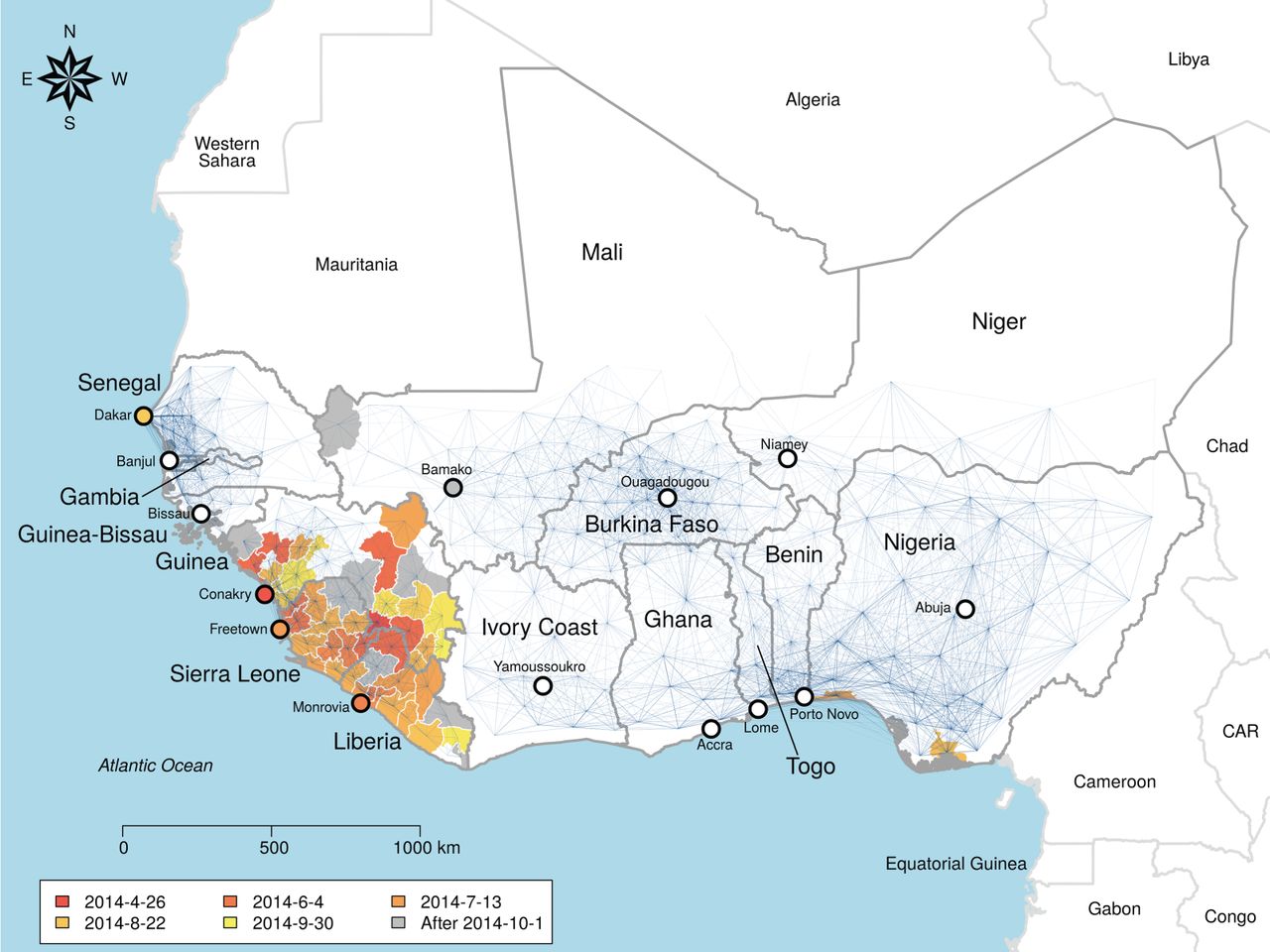
Spatial spread of Ebola
The Odum School’s Drew Kramer led a study on the recent spatial spread of Ebola in West Africa, and former lab member Laura Alexander and Andrew were contributors to this work, which was recently published in the Royal Society’s Open Science journal. Among the findings were that a gravity model provided a good description of the spread. This means that transmission between towns depended on the population size of each, and their distance from each other. Additionally, if one town was inside the hotspot of Guinea, Liberia and Sierra Leone and the other was outside, then risk of transmission out of the core region was lower, due to border closures. Individual movement patterns from cell phone data did not provide a competitively good fit. Counterfactual scenario building showed that the initial town infected would have a large effect on the regional extent of the spread of Ebola. As well as highlighting the importance of integrated geography in controlling epidemic processes, the study provides methods to predict vulnerable towns and those where intervention may have the most impact.
The big picture of infectious diseases
Macroecology is big. Spatially, temporally, taxonomically. Andrew is part of working group applying ideas from macroecology to host-parasite data. To kick things off, the group has written a review/synthesis paper explaining the goals and outlining the challenges and opportunities that lie ahead. It’s here.
Sexual transmission of Ebola virus may jeopardize outbreak control
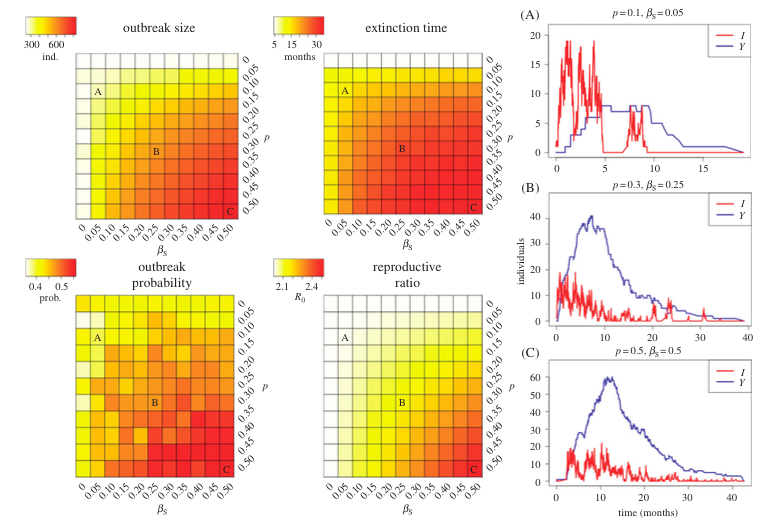
Evidence has shown that Ebola virus may remain in semen of survivors for several months after the virus has been cleared from the blood. To investigate the effect that sexual transmission can have on disease dynamics, we developed a mathematical model incorporating a convalescent compartment capable of infecting individuals through sexual contact. The model was developed and partially parameterized using convenience survey data of survivors. However, uncertainty in the parameters for the sexual transmission rate and probability of becoming sexually infectious led to varying these parameters within likely ranges.
We found that outbreaks brought under control (through behavior changes that reduced direct transmission) had an attack ratio (proportion ultimately infected) of ~25%, with sexual transmission increasing the attack ratio to 80%. Sexual transmission also increased the average duration of outbreaks, the probability of outbreaks occurring and the reproductive ratio of the parasite (increasing it from 2.0 to ~2.5). The model demonstrated how broken chains of direct transmission were repaired through sexual transmission resulting in flare-ups late in the outbreak.
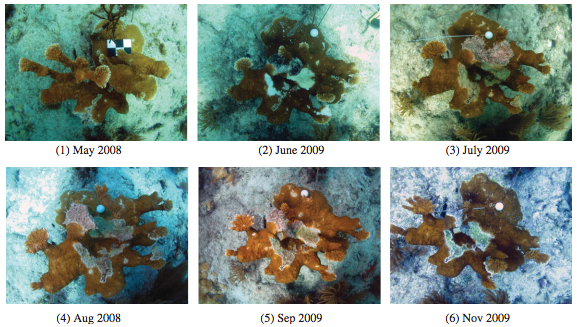
Coral diseases under the microscope
As part of large group, lab members Ashton, Brett and Andrew have been exploring some of the challenges in identifying causal agents of disease affecting coral populations. The host species is elkhorn coral, which has been experiencing alarming declines in recent times. These studies help to develop surveillance, diagnostic and modeling approaches to learn as much as we can about this important reef-building coral species.
- Joyner et al. 2015, Applied Environmental Microbiology, “Systematic analysis of white pox disease in Acropora palmata of the Florida Keys and the role of Serratia marcescens“
- Sutherland et al. 2016, Philosophical Transactions of the Royal Society of London B, “Shifting white pox etiologies affecting Acropora palmata in the Florida Keys, 1994-2014“
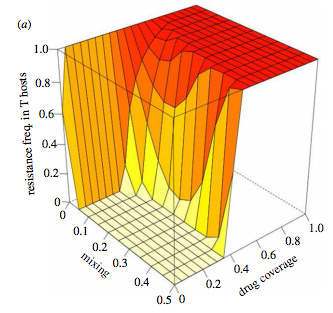
Refugia and drug resistance
MRSA and extensively drug-resistant TB are just two examples where we’re running out of solutions to combat pathogens. In animal health, refugia are increasingly discussed as a management strategy. Refugia are untreated subpopulations – they provide a safe haven for pathogens. By connecting refugia with drug-treated populations there is hope to limit the spread of drug resistance at an acceptable cost in terms of disease burden. Basically, the connections let drug-susceptible pathogens into the treated population to stop their drug-resistant counterparts taking over. We built a model to try and clarify when this sort of strategy might work (Park et al. 2015, Biol. Lett.). We found that the anticipated outcome (strong connections = high prevalence and low resistance in treated groups) was too simplistic. Rather, there are epidemiological and evolutionary interactions at work and these can be understood by decoupling transmission and selection through mathematical analysis.
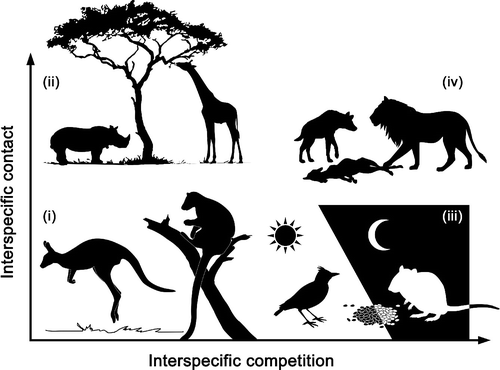
Interspecific contact and competition may affect the strength and direction of disease-diversity relationships for directly transmitted microparasites
With global declines in species diversity, understanding the effects of host species loss on disease dynamics, and parasite transmission, is vital. In recent reviews by Civittello et al. (2015) and Johnson et al. (2015), the authors call for a more mechanistic understanding of disease diversity relationships, which generally posit that increases in host diversity result in a decrease in disease risk to host species.
We’ve developed a framework to explore the disease diversity relationships for directly transmitted, multi-host microparasites, integrating host regulation through intra- and interspecific competition, which can be dependent or independent on interspecific contact rates.
The results of our study show that
- a decrease in parasite fitness does not necessarily result from host regulation via interspecific competition
- an increase in host diversity does not necessarily result in a decrease of frequency dependent parasite transmission
Overall, we highlight that species identity and their ecological interactions jointly determine the outcome of microparasite transmission in multihost communities.
(How) does vector species richness increase parasite transmission?
 This is a question we got interested in through hemorrhagic disease, a vector-borne viral disease affecting white-tailed deer. We observed that disease reporting rate was positively associated with vector species richness (VSR: where species of midge belong to the large genus Culicoides). We thought this could be because high VSR:
This is a question we got interested in through hemorrhagic disease, a vector-borne viral disease affecting white-tailed deer. We observed that disease reporting rate was positively associated with vector species richness (VSR: where species of midge belong to the large genus Culicoides). We thought this could be because high VSR:
- is associated with high vector abundance
- increases the likelihood that the vector community contains a highly competent subset
- is associated with long seasonal transmission if vector species in a community exhibit distinct phenologies
We found no support for 1 and 2 in our system, but strong support for 3. This means that VSR can manifest as functional diversity, facilitating parasite transmission. It remains to be seen if this operates more widely in vector borne diseases.